Title: Unveiling the Atomic Architecture and Industrial Potential of Graphite: A Material Powering Modern Innovations
By CFCCarbon Editorial Team | Updated: May 18, 2025
Introduction
Graphite, a crystalline allotrope of carbon, is far more than the “lead” in pencils. Its unique layered atomic structure underpins its role as a critical material in industries ranging from energy storage to aerospace. This article explores the structural intricacies of graphite, its production methods, diverse applications, and cutting-edge advancements shaping its future.

isostatic graphite rods blocks plates material manufacturer factory China
1. Atomic Structure: The Foundation of Graphite’s Properties
Graphite’s structure comprises stacked layers of carbon atoms arranged in hexagonal lattices. Each layer, termed a graphene sheet, features sp²-hybridized carbon atoms bonded covalently in a planar honeycomb pattern with a bond length of 1.422 Å11. The layers are held together by weak van der Waals forces (0.335 nm apart), enabling easy interlayer sliding—a property central to its lubricity.
Crystal Forms
-
Hexagonal (ABAB): The most common form, where layers alternate in alignment.
-
Rhombohedral (ABCABC): A less stable variant that reverts to hexagonal stacking at high temperatures.
-
Turbostratic: Disordered stacking, often found in synthetic graphite, impacting electrical and thermal conductivity.
Defects such as vacancies, dislocations, and edge irregularities (dangling bonds) influence mechanical strength and reactivity. Edge defects often bind functional groups (e.g., -OH, -O), altering surface chemistry.
2. Production Methods: From Natural Deposits to Synthetic Innovations
Natural Graphite
Mined in three primary forms:
-
Flake: High-purity crystalline graphite used in batteries and electronics.
-
Amorphous: Lower carbon content, derived from metamorphosed coal, ideal for refractories.
-
Vein: Rare, ultra-pure deposits critical for high-performance applications.
Synthetic Graphite
Produced via energy-intensive processes:
-
Acheson Process: Traditional method involving petroleum coke heated above 2,500°C1.
-
Catalytic Graphitization: A breakthrough by Texas A&M reduces temperatures to 1,400°C using iron catalysts, slashing energy use by 50% and enabling sustainable production from refinery byproducts.
3. Industrial Applications: Beyond Lubrication
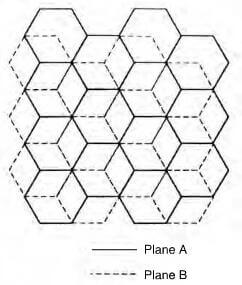
schematic of hexagonal graphite crystal
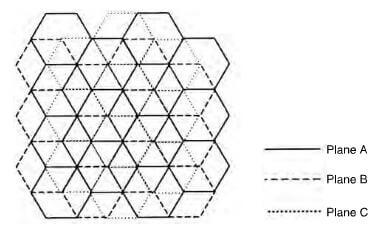
schematic of rhombohedral graphite crystal
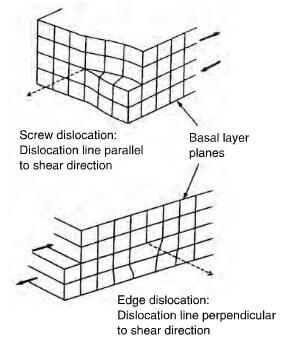
shear disiocations in a graphite crystal
Energy Storage
-
Lithium-Ion Batteries: Graphite dominates anode materials, storing lithium ions between its layers. Coated spherical graphite (99.95% purity) minimizes expansion during cycling, while synthetic variants enhance cycle life.
-
Fuel Cells: High-purity graphite electrodes facilitate efficient ion transport.
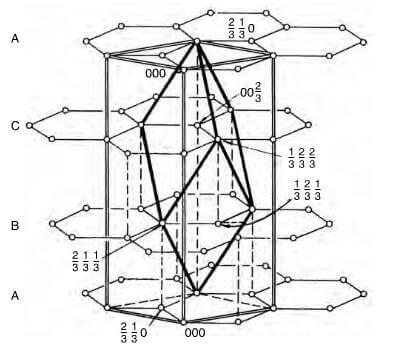
Rhombohedral unit cell structure of graphite
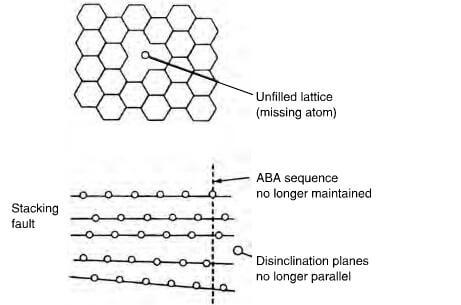
Schematic of crystallite imperfections in graphite showing unfilled lattice, stacking fault and disinclination.
Semiconductor Manufacturing
-
Heaters & Crucibles: Graphite’s thermal stability (melting point >3,600°C) ensures uniform heating for silicon wafer growth.
-
Etching Electrodes: Synthetic graphite resists plasma corrosion in etching machines.
Metallurgy & Aerospace
-
Refractories: Graphite-lined furnaces withstand extreme temperatures in steelmaking2.
-
Composite Materials: Carbon-fiber-reinforced graphite reduces aircraft weight while maintaining strength.
4. Cutting-Edge Research & Sustainability
Biographite
New Zealand researchers convert forestry waste into graphite via thermo-catalytic methods, reducing reliance on mining.
Nano-Graphite
Nanostructured graphite sheets exhibit exceptional surface area and conductivity, promising advances in supercapacitors and catalysts.
Silicon-Graphite Hybrids
While silicon anodes offer higher energy density, their 400% expansion during cycling remains a hurdle. Blending silicon with graphite (1–3%) balances performance and durability.
5. Challenges and Future Outlook
-
Environmental Impact: Graphite mining raises ecological concerns, driving demand for synthetic and bio-based alternatives.
-
Supply Chain Resilience: China dominates production (75% global output), prompting investments in domestic synthetic graphite facilities.
-
Electrification Trends: Rising EV adoption could increase graphite demand tenfold by 2030, necessitating scalable, low-carbon production methods.
Conclusion
Graphite’s atomic architecture—a symphony of covalent layers and weak interplanar forces—enables its versatility across industries. As innovations like catalytic graphitization and nano-engineering redefine its potential, graphite remains indispensable in the transition to renewable energy and advanced manufacturing.
Explore CFCCarbon’s high-purity graphite solutions for aerospace, energy, and semiconductor applications at www.cfccarbon.com.
related news /articles:
The forms of carbon (1)- Diamond-Guide to Types, Production, and Applications
Refractory high temperature resistant material: graphite insulation felt
Resin impregnated graphite material, a kind of mechanical seal bearing
Development concerns of Carbon-carbon composites as recuperator materials (4)