The physical properties reviewed in the previous section are essentially unaffected by the size and orientation of the crystallites in the aggregate. As a result, they can be considered valid for all forms of graphite. This is no longer true for some of the properties listed in this and the following sections, and these properties may vary considerably depending on crystallite size and orientation and other factors related to the processing conditions.
The thermal properties are summarized in Table 3.4. Whenever possible, a range of property values is given. More detailed values are given in subsequent chapters.
Table 3.4. Theoretical thermal properties of graphite
Heat of combustion △Hco @ 25C and 393.13
Constant pressure to form CO2 gas, kJ/mol
Standard entropy S° at 25C, J/mol.K 5.697-5.743
Entropy △S298, J/mol.K 152.3
Enthalpy △H298, kJ/mol 716.88
Specific heat @ 25C, kJ/kg.K (see bellow) 0.690-0.719
Thermal conductivity @ 25C, W/m.K ab directions 398
C direction 2.2
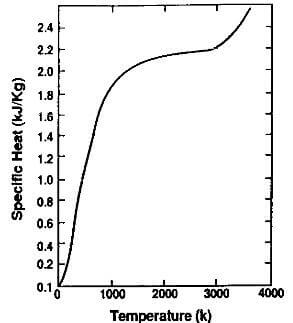
Fig.3.8-specific heat of graphite vs. temperature at one atmosphere
Thermal expansion: see bellow
Heat capacity (Specific heat): The molar heat capacity of graphite is reported as 8.033-8.635 J/mol.K at 25C. As with all elements, it increases with temperature, with the following relationship (T in deg K).
Cp=4.03+(1.14 ×10-3)T – (2.04 ×105)/T2
The specific heat increases rapidly with temperature, up to 1500K where it levels off at approximately 2.2 kj/kg.K
as shown in Fig.3.8. It is believed to be relatively insensitive to the differences between the various grades of synthetic graphite, and the spread of values found in the literature may be attributed to experimental variations. The average value is compared to that of selected other elements in Table 3.5. As can be seen, it is higher than most metals.
Table 3.5. Specific heat of selected elements
Cp at 25C and 1 atm.
(kJ/kg.K)
Graphite 0.690-0.719
Diamond 0.502-0.519
Boron 1.025
Aluminum 0.900
Tianium 0.523
Copper 0.385
Niobium 0.263
Rhenium 0.242
Tungsten 0.130
Water 4.186