The next two terms, 2s2 and 2p2 refer to the four electrons in the L shell. The L shell, when filled, can never have more than eight electrons. The element neon has a filled L shell. The L-shell electrons belongs to two different subshells, the s and the p, and the 2s and the 2p electrons have different energy levels. The two 2s electrons have opposite spin and the two 2p electrons parallel spin. This view of the carbon atom is represented schematically in Fig. 2.1.
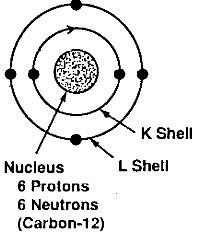
carbon-12
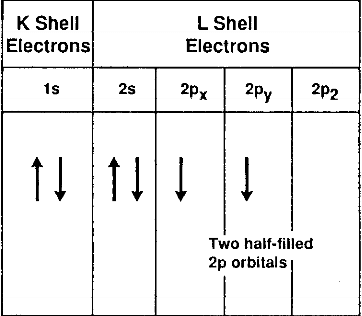
Fig.2.1-schematic of the electronic structure of the carbon atom in the ground state
The configuration of the carbon atom described above refers to the configuration in its ground state, that is, the state where its electrons are in their minimum orbits, as close to the nucleus as they can be, with their lowest energy level.
In any given atom, the electrons located in the outer orbital are the only ones available for bonding to other atoms. These electrons are called the valence electrons. In the case of the carbon atom, the valence electrons are the two 2p orbitals. Carbon in this state would then be divalent, since only these two electrons are available for bonding.
Divalent carbon does indeed exist and is found in some highly reactive transient-organic intermediates such as the carbenes. However, the carbon allotropes and the stable carbon compounds are not divalent but tetravalent, which means that four valence electrons are present. How this increase in valence electrons occurs is reviewed in sec. 3.0.
The carbon valence electrons are relatively easily removed from the carbon atom. This occurs when an electric potential is applied which accelerates the valence electron to a level of kinetic energy which is enough to offset the binding energy of this electron to the atom. When this happens, the carbon atom becomes ionized forming a positive ion. The measure of this binding energy is the ionization potential, the first ionization potential being the energy necessary to remove the first outer electron, the second ionization potential, the second outer second electron, etc. The ionization energy is the product of the elementary charge and the ionization potential, expressed in electron volts, eV.
The first ionization potentials of carbon and other atoms close to carbon in the Periodic Table are listed in Table 2.2. It should be noted that the ionization energy gradually increases going from the first element of a given shell to the last. For instance, the value for lithium is 5.39V and for neon, 21.56V. It is difficult to ionize an atom with a complete shell such as neon, but easy to ionize one with a single-electron shell such as lithium.
As shown in Table 2.2 above, carbon is located half-way between the two noble gases, helium and neon. When forming a compound, carbon can either lose electrons and move toward the helium configuration, or it can gain electrons and move toward the neon configuration.
The six ionization potentials of the carbon atom are shown in Table 2.3.
Table 2.3. lonization Potentials of the carbon atom
| Number | Shell | Orbital | Potential, V |
| 1st | L | 2p | 11.260 |
| 2nd | L | 2p | 24.383 |
| 3d | L | 2s | 47.887 |
| 4th | L | 2s | 64.492 |
| 5th | K | 1s | 392.077 |
| 6th | K | 1s | 489.981 |
As shown in Table 2.3., in an element having a low atomic number such as carbon, the difference in energy of the electrons within one shell, in this case between the 2s and 2p electrons, is relatively small compared to the differences in energy between the electrons in the various shells, that is between the K shell. As can be seen, to remove the two electrons of the K shell requires considerably more energy than to remove the other four electrons.