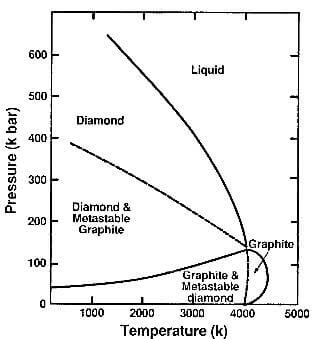
The carbon phase diagram is shown in Fig.2.20. Another expression of the T-P phase diagram, showing the calculated total vapor pressure of carbon, is shown in Fig.3.7 of ch. 3.
carbon phase diagram
Carbon vaporizes at 4800K at pressure of 1000 atmospheres, which is the area where diamond is stable. The high-pressure conversion of diamond from graphite occurs at temperatures of approximately 3000K and pressures above 125Kbars and will be reviewed in Ch. 12.
Allotropic forms: In the preceding sections, the various ways that carbon atoms bond together to form solids were reviewed. These solids are the allotropes of carbon, that is, they have the same building block- the element carbon- but with different atomic hybrid configurations: sp3, sp2 or sp.
These allotropic solids can be classified into three major categories: (1) the sp2 structures which include graphite, the graphitic materials, amorphous carbon, and other carbon materials. (2) the sp3 structures which include diamond and lonsdaleite, reviewed in Ch.11. and (3) the fullerenes.
These allotropes are sometimes found in combination such as some diamond-like carbon (DLC) materials produced by low-pressure synthesis, which are actually mixtures of microcrystalline diamond and graphite.
Recent investigations have revealed the existence of a series of diamond polytypes such as the 6-H hexagonal diamond. The structure and properties of these polytypes are reviewed in Ch. 11. Also under investigation is a hypothetical phase of carbon based on a three-dimensional network but with sp2 bonds. This phase could be harder than diamond, at least in theory. A carbon phase diagram incorporating these new polytypes has yet to be devised.
The fullerene carbon molecules: The recently discovered family of fullerene carbon molecules are considered another major allotropic form of carbon that combines both sp2 and sp3 bonds. These molecules are still in the early stages of investigation and it will be some time before practical applications are found. The fullerenes are reviewed in Ch. 15.