The Allotropes of Carbon: Structures, Applications, and Innovations
By CFCCarbon Composite Manufacturer | May 18, 2025
Carbon, one of the most versatile elements in the periodic table, forms diverse allotropes—distinct structural forms of the same element.

high purity isostatic graphite rods rounds D100 material manufacturer in China 08 (3)
These allotropes exhibit unique physical and chemical properties, enabling revolutionary applications across industries. From the ancient uses of graphite to cutting-edge nanomaterials like graphene, carbon’s adaptability continues to redefine technological boundaries.
1. What Are Carbon Allotropes?
Allotropes are different structural forms of an element, arising from variations in atomic bonding. Carbon’s ability to hybridize its orbitals (sp³, sp², sp) allows it to form covalent networks with distinct geometries. The most well-known allotropes include diamond, graphite, fullerenes, carbon nanotubes (CNTs), and graphene.
2. Key Allotropes and Their Structures
Diamond
-
Structure: A 3D tetrahedral lattice where each carbon atom forms four strong sp³ bonds (bond length: 0.154 nm).
-
Properties:
-
Extreme hardness (highest among natural materials).
-
High thermal conductivity but poor electrical conductivity.
-
Transparent with a high refractive index.
-
-
Applications: Industrial cutting tools, high-pressure experiments (diamond anvils), and emerging uses in quantum computing (via nitrogen-vacancy centers).
Graphite
-
Structure: Layers of sp²-bonded carbon atoms arranged in hexagonal rings (bond length: 0.142 nm), held together by weak van der Waals forces.
-
Properties:
-
Soft, slippery, and electrically conductive due to delocalized π-electrons.

carbon graphite felt insulation soft hard rigid board short long fiber chopped material manufacturer factory China (s)
-
Thermally stable up to 3,600°C.
-
-
Applications: Lubricants, electrodes in batteries, and nuclear reactor components.
Graphene
-
Structure: A single layer of graphite, forming a 2D hexagonal lattice.
-
Properties:
-
Thinnest known material (one atom thick).
-
Exceptional tensile strength, flexibility, and electrical conductivity.
-
-
Applications: Flexible electronics, advanced composites, and energy storage devices.
Fullerenes and Carbon Nanotubes
-
Fullerenes: Spherical molecules (e.g., C60 “buckyballs”) with mixed sp²/sp³ bonding. Used in drug delivery and organic photovoltaics.
-
CNTs: Cylindrical structures with sp² bonds. Applications include lightweight composites, sensors, and thermal management systems.
- The relationship between the allotropes of carbon is shown in figure 2.1 bellow:
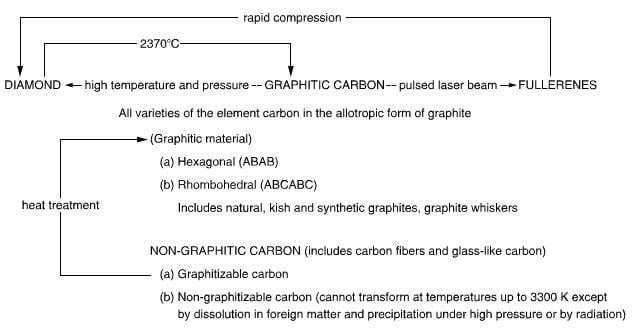
the allotropes of carbon
3. Production Methods
-
Diamond: Synthesized via high-pressure high-temperature (HPHT) processes or chemical vapor deposition (CVD).

3D 4D carbon fiber composite material manufacturer factory -airplane brake disc plates sheets U L profiles-2D(s)
-
Graphite: Mined naturally or produced by heating petroleum coke.
-
Graphene: Produced through mechanical exfoliation, CVD, or chemical reduction of graphite oxide.
-
Fullerenes/CNTs: Generated via arc discharge or laser ablation techniques.
4. Industrial and Technological Applications
Aerospace & Automotive
-
Carbon Fiber Composites: Lightweight, high-strength materials derived from graphite precursors. Used in aircraft and electric vehicles.
-
Diamond-Coated Tools: Enhance precision in machining aerospace alloys.
Electronics
-
Graphene Transistors: Enable faster, energy-efficient microchips.
-
CNT-Based Sensors: Detect gases and biomolecules with high sensitivity.
Energy
-
Graphite Anodes: Critical for lithium-ion batteries.
-
Diamond Heat Spreaders: Improve thermal management in high-power electronics.
Healthcare
-
Nanodiamonds: Used in targeted drug delivery and bioimaging.
-
Fullerene Derivatives: Explore antioxidant and antiviral therapies.
5. Innovations and Future Prospects
-
Diamane: A 2D diamond film with potential in nanoelectronics1.
-
Carbon Peapods: CNTs encapsulating fullerenes for quantum computing applications.
-
Sustainable Synthesis: Green methods for producing graphene and CNTs to reduce environmental impact.
6. Why Choose CFCCarbon Products?
At CFCCarbon, we specialize in advanced carbon-based composites tailored for high-performance applications. Our offerings include:
-
Customized Graphite Components: For high-temperature furnaces and energy storage systems.
-
Diamond-Coated Tools: Engineered for durability in extreme conditions.
-
Graphene Solutions: Enhancing conductivity in flexible electronics.
Explore our innovations at www.cfccarbon.com.
Conclusion
The allotropes of carbon—from diamond’s unrivaled hardness to graphene’s revolutionary conductivity—underscore carbon’s unparalleled versatility. As research advances, these materials will drive breakthroughs in sustainability, healthcare, and next-gen technologies. Stay ahead with CFCCarbon’s cutting-edge solutions.
For more details on carbon composites and custom orders, visit CFCCarbon.
related news /articles:
The forms of carbon (1)- Diamond-Guide to Types, Production, and Applications
Ultrahigh temperature coatings on carbon fiber carbon composites
The carbon element and its various forms