Graphite is composed of series of stacked parallel layer planes shown schematically in Fig.3.1, with the trigonal sp2 bonding described in Ch.2.sec.4.0. In fig 3.1, the circles showing the position of the carbon atoms do not represent the actual size of the atom. Each atom, in fact, contacts its neighbors.
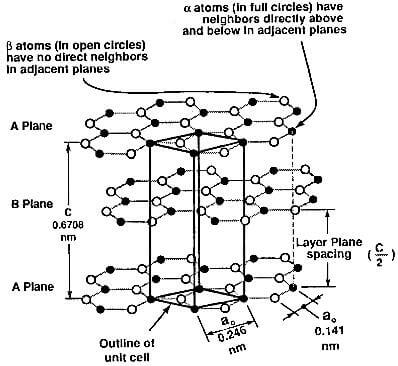
Fig.3.1-crystal structure of graphite showing ABAB stacking sequence and unit cell.
Within each layer plane, the carbon atom is bonded to three others, forming a series of continuous hexagons in what can be considered as an essentially infinite two-dimensional molecule. The bond is covalent and has a short length and high strength. The hybridized fourth valence electron is paired with another delocalized electron of the adjacent plane by a much weaker van der waals bond of only 7 kj/mol. Carbon is the only element to have this particular layered hexagonal structure.
The spacing between the layer planes is relatively large or more than twice the spacing between atoms within the basal plane and approximately twice the van der waals radius of carbon. The stacking of these layer planes occurs in two slightly different ways: hexagonal and rhombohedral.
Hexagonal graphite: The most common stacking sequence of the graphite crystal is hexagonal with a –ABABAB- stacking order, in other words, where the carbon atoms in every other layer are superimposed over each other as shown in Fig.3.1.
Atoms of the alpha type, which have neighbor atoms in the adjacent planes directly above and below, are shown
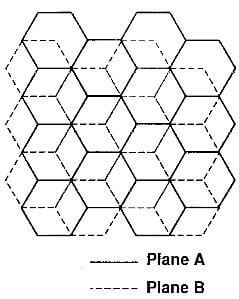
Fig.3.2-schematic of hexagonal graphite crystal
with open circles. Atoms of the beta type, with no corresponding atoms in these planes, are shown with full circles. A view of the stacking sequence perpendicular to the basal plane is given in Fig.3.2.
Other characteristics of the graphite crystal are the following:
- The crystallographic description is given by the space group D46H-P63/mmc.
- The crystal lattice parameters, i.e., the relative position of its carbon atoms
- The common crystal faces are 0002, 1010, 1001 and 1012.
- The crystal cleavage is 0002 with no fracture.
- The crystal is black and gives a black streak.
- Hexagonal graphite is the thermodynamically stable form of graphite and is found in all synthetic materials.
- Rhombohedral graphite: The other graphite structure is rhomobohedral with the stacking order –ABCABCABC-. The carbon atoms in every third layer are superimposed. The
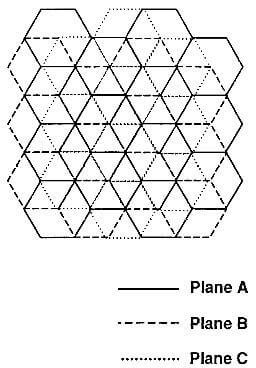
Fig.3.3-schematic of rhombohedral graphite crystal
crystallographic description is given by the space group D53d-R3m. The crystal lattic parameters are ao=0.2256 nm and co=1.006 nm. A view of the stacking sequence perpendicular to the basal plane is given in Fig.3.3
Rhombohedral graphite is thermodynamically unstable, and can be considered as an extended stacking fault of hexagonal graphite. It is never found in pure form but always in combination with hexagonal graphite, at times up to 40% in some natural and synthetic materials. It usually reverts to the hexagonal form during heat treatment above 1300C. It should be noted that in both structures, hexagonal and rhombohedral, no basal plane lies directly over another one.