Now, we will discuss the presence of sharp current peaks and dips at the edges of HOPG. In Fig.8(a), 8(b), and 8(c) we motivate schematically the step edges of different kinds that can give rise to the corresponding current vs distance (x) profile across the step edges and layers. Certain sheets/edges show a sharp rise in the conductance. As mentioned in the Introduction, there are two types of edges formed on graphene sheets, namely, zigzag and armchair. During the tearing process of the graphite sheets to produce ribbons and steps, the sheets are torn forming these two edges. Earlier detailed theoretical calculations have shown that zigzag edges have edge states
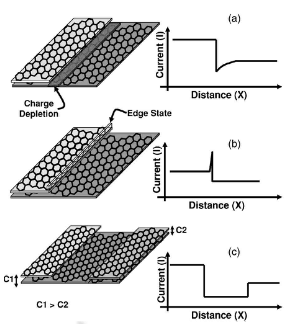
Fig.8-step edges of different kind can give rise to the current vs distance across the step edge and layers
localized at the edges having a higher electron density. Hence these edges will have higher electrical conductance. Thus during the mapping of the local conductance these edges will shown up as the brighter streaks. During the tearing process there is equal probability that the edges formed will be of either zigzag type or armchair type. The same edge can contain both types of edge shapes. This can also happen due to the mosaicity of the graphite sheet. Each mosaic can be oriented in different directions and during the tearing process it will tear along any suitable direction.
As mentioned earlier the armchair edges have no edge states and are thus less conducting compared to the zigzag edges. It is not clear whether reconstruction is taking place at the edges. We can only say that the appearance of a bright streak or disappearance of it depends on the shape of the edge. Very recently, a similar conclusion was drawn using STM and scanning tunneling spectroscopy.
The peculiar dip in current observed at some edges is puzzling, i.e., the formation of a charge depleted region. As we have argued earlier, the interlayer bonding utilizes the π orbitals to a certain extent. Near the step edge if there are no excess charges then, it seems like the electrons from the π orbitals of the underlying layer have been dragged from the vicinity of the edge causing the depletion of charge in that region. If the ribbons are very loosely held then this effect diminishes or is not observed. This observation was not reported earlier and will need more theoretical understanding of the role of π orbitals in the interlayer coupling apart from its role in electrical conduction.
We have demonstrated that AFM with conducting tip can also be used and in certain cases may produce better results than STM in the measurement of local conductivity mapping at the cost of spatial resolution. From the present study we could say that π orbitals may play an important role in the interlayer forces apart from electrical conductance. When the top layers of the graphite sheets are loosely held with the bulk graphite, then the conductivity of the layer increases. The increase in the conductivity of these sheets can be explained only by assuming that the π electrons on the loosely held sheets are not much involved in binding between the layers and hence would have higher mobility. Additionally, crumpling of top layers wherever present will also create additional carriers in the plane. A sharp increase or absence in conductance current at the edges of the graphite sheets have been attributed to either zigzag shape edges or armchair shape edges, respectively. We have also observed the presence of these two types on a single edge of graphite sheet. In this article, a charge depleted region near the step edge on the lower layer graphite sheet is reported.
We think that all the above experimental observations are very important and will require more theoretical understanding and will have an impact on the understanding of the graphene layers.