Graphite has very good chemical resistance and is used for the construction of special chemical plants such as heat exchangers.
Graphite will form carbides with B, Si, V, Fe, Mo, W and Ta. Oxidizing gases such as oxygen (>150C), air (>250C) and steam (>300C) will attach graphite and such media can be used to manufacture activated carbon. A more graphitic carbon with fewer active sites will be less active.
Graphite reacts to form intercalation compounds when the compound enters between the graphite layer planes, forcing them apart, although heating tends to remove the entire intercalated product, when the graphite reverts to its normal form. Although the layer planes are pushed aprt, the distance between the carbon atoms within a sheet remains unaltered. Liquid potassium can form C8K (figure 2.28) and C16K with the interlayer spacing increased from 0.335 to 0.54nm.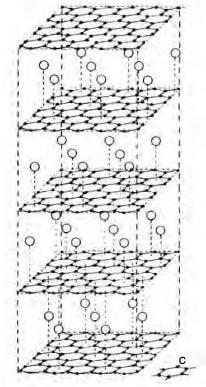
Sulfuric acid nitric acid to is a strong oxidizing agent and forms a purple-blue bisulfate, C24+(HSO4)-(H2SO4)2, swelling the graphite with an interlayer spacing of 0.798nm. This process can be used to purify graphite and to make graphite foil and colloidal graphite. More than sixty halides are known to intercalate graphite, such as FeCl3 at 180C, which gives an interlayer spacing of 0.94nm.
Heating with a strong oxidizing mixture of potassium chlorate/nitric acid/ sulfuric acid, graphite forms what can be termed graphite oxide, when oxygen and hydrogen are taken up to give a green-brown product C8O2(OH)2 (where most of the hydrogen is in the form of water) with an interlayer spacing of 0.6-1.1 nm.