Pure graphite is one of the most chemically inert materials. It is resistant to most acids, alkalies and corrosive gases. However impurities are almost always present to some degree in both natural and artificial graphites and often have an important catalytic effect with resulting increase in the chemical reactivity.
The anisotropy of the graphite crystal is reflected in its chemical behavior. Reaction with gases or vapors occurs preferentially at “active sites”, i.e., the end of the basal planes of the crystal which are the zigzag face and the arm-chair face as shown in fig.3.14, and at defect sites, such as dislocations, vacancies, and steps. Reaction with the basal plane surfaces in far slower. The reason is that the graphite crystal exhibits large differences in surface energy in the different crystallographic directions; these energies amount to 5 J/m2 in the prismatic plane but only 0.11 J/m2 in the basal plane. These differences account for the different rate of reaction, i.e., slow at the basal plane and rapid at the edge surfaces found at the termination of the basal planes or at defects within the basal plane. Consequently, graphite materials with large crystals and few defects have the best chemical resistance.
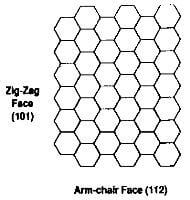
the faces of a graphite crystal
The chemical reactivity is also appreciably affected by the degree of porosity, since high porosity leads to large increase in surface area with resulting increase in reactivity. Differences in reactivity between one form of graphite or another can be considerable. Obviously, high surface area materials such as activated carbon are far more reactive than dense, pore-free or closed-pore materials such as glassy carbon.
Reactivity also generally increases with increasing temperature and, at high temperatures, graphite becomes far more reactive. For instance, above 450C, it oxidizes readily with water, oxygen, some oxides, and other substances.
In this article, only the general chemical behavior of graphite will be considered. Reviews of the chemical reactivity of specific graphite materials will be given in subsequent chapters.
Reaction with oxygen and hydrogen: The one major exception to the generally excellent chemical resistance of graphite is poor resistance to the elements of Column VI, particularly oxygen and oxygen compounds. Oxidation begins in air at 350-400C. This low-temperature oxidation is in contrast with the behavior of other refractory materials: oxides, for instance, do not oxidize and many carbides form a protective oxide film on their surface that delays oxidation. In contrast, the oxides formed by the oxidation of graphite are gaseous and offer no protection to the surface.
As mentioned in Sec. 7.1 above, the reaction rate is site preferential and oxidation is much higher along the zig-zag face of the crystal than it is along the armchair face. Oxidation can be hindered by increasing the degree of graphitization and the crystallite size.
The oxidation of graphite and the available protective coatings are reviewed in Ch.9. The controlled oxidation of graphite, known as activation, results in open structures with extremely high surface area.
Graphite does not react with hydrogen at ordinary temperatures. It reacts in the 1000-1500C range to form methane. The reaction is accelerated in the presence of a platinum catalyst. With nickel catalyst, the reaction begins at approximately 500C.
Reaction with metals: Graphite reacts with metals that form carbides readily such as the metal of groups IV, V and VI. These carbides are the so-called hard carbides, which include the carbides of tungsten, molybdenum, titanium, vanadium and tantalum, as well as the non-metal carbides of silicon and boron.
Graphite reacts with iron to form iron carbides, Fe3C, usually by the direct solution of carbon in the molten iron. Iron carbide may also be formed from the reaction of iron with a carbon-containing gas. This reaction is known as case-hardening.
The reaction rate of graphite with the precious metals, aluminum, and the III-V and II-VI semiconductor compounds is low and graphite is used successfully as a crucible to melt these materials.
Graphite reacts readily with the alkali metals: potassium, calcium, strontium, and barium. The atoms of some of these metals, notably potassium, can readily penetrate between the basal planes of the graphite crystal to form intercalated with useful properties. These compounds are reviewed in Ch.10, sec.3.0.
Reaction with halogens, acids, and alkalis: Like the alkali metals, some halogens, particularly fluorine, form intercalated compounds with graphite crystals. Reaction usually starts at 600C. However, graphite does not react with chlorine at temperatures below that of the electric arc.
Oxidizing acids attack graphite to varying degree depending on the nature and surface area of the material. The reaction with concentrated nitric acid is as follows:
C + 4HNO3 → 2H2O + 4NO2 + CO2
Depending on the reaction conditions, other products may be formed such as graphitic oxide, mellitic acid and hydrocyanic acid.
Another oxidizing acid that attacks graphite is boiling sulfuric acid. The simplified reaction is the following:
C + 2H2SO4 → CO2 + 2H2O + 2SO2
Other by-products may be formed such as benzoic acid, C6H5CO2H, and mellitic acid, C6(CO2H)6.
Hydrofluoric acid and the alkali hydroxides generally do not react with graphite.