During aircraft operation, alkali-metal salts from marine environments and de-icing fluids can access the surface of the C-C composite materials within the aircraft braking system and act as catalysts for the oxidation reaction and dramatically accelerate the oxidation rate. Catalysts based on sodium and potassium salts have been reported to be more effective and can lead to oxidation increases in excess of an order of magnitude. This is highly alarming when considering the fact that oxidation experiments in the presence of K2O3 catalyst have shown a large drop in the compression strength even after weight loss values of 2.5% and 5%. The study by Duvivier et al. has also demonstrated that the increase in the rate of oxidation as a result of catalysis with K2O3 is dependent on the catalyst content up to about 0.80%; above this figure, the rate of oxidation remains more or less constant. Therefore since only a small amount of these catalyst compounds is sufficient to cause a significant increase in the oxidation rate, it is of paramount importance to gain as detailed as possible, an understanding of the catalytic oxidation reaction mechanism in order to devise the means to slow down, inhibit or prevent the catalytic oxidation. The enhancement of carbon gasification/oxidation has been the object of extensive studies in the last few decades owing to its use as a fossil fuel. Important insights were thus gained from these earlier studies on the catalytic effect of alkali-metals on the oxidative process. The catalytic effect of the oxides or carbonates of potassium, sodium and calcium on the oxidation of C-C composites and carbon as a fossil fuel has been reported by several investigators. Some other important papers related to fossil fuel carbon are also reviewed here as they bear relevance to the understanding or the evolution of the understanding of the catalytic mechanism.
Adjorlolo and Rao investigated the catalytic effect of K2O3 and Na2CO3 during the gasification of metallurgical coke in CO2. The effectiveness of both catalysts throughout the oxidation reaction implied that some mechanism existed for the fast inward diffusion of the catalysts. In fact this observation is very similar to the behaviour of the catalysts during the oxidation of C-C composites. Since the melting point of the carbonates/oxides and of the constituent metals had not been exceeded, the authors adopted the following vapour-cycle mechanism originally proposed by Fox and White to interpret the catalytic mechanism:
M2CO3(s)+ 2C → 2M(g) + 3CO(g) (5)
2M(g) + 2CO2(g) → M2CO3(s) +CO(g) (6)
Whereby the alkali metal vapour is produced at catalyst/carbon junctions and gets reconverted to the carbonate upon contact with CO2. This mechanism involves formation of gaseous sodium or potassium and can mechanistically explain the movement of the catalyst deeper into the carbon samples or the C-C composite. Thus it can explain why the catalyst can remain active throughout the entire period of oxidation. At the same time, it would be reasonable to also gradually expect some loss of the catalyst due to vapourisation of the corresponding metals. Rao et al. also calculated the respective thermodynamic driving force for catalysis for each carbonate by obtaining the difference in the partial pressures of sodium and potassium for the two reactions above that were proposed to be involved in the mechanism of catalysis. The higher difference for potassium led them to propose that K2CO3 was a more effective catalyst than Na2CO3 was observed to lead to a lower activation energy value than K2CO3. This observation is rather unusual as in virtually all of the other investigations, K2CO3 has been observed to have a greater catalytic effect. As metallurgical coke by nature varies in composition and also contains several other oxides/carbonates and metals that may have a catalytic effect on gasification, it is possible that a more oxidizing synergistic effect may have been active in the case of Na2CO3 in their investigation. In another study, Zahedi and Miller discussed various potential mechanisms to explain the catalytic gasification route for carbon in the presence of K2CO3. These included the redox cycle involving decomposition and reformation of the carbonate on the carbon surface and involved alkali oxides and hydroxides as intermediate compounds similar to the mechanism proposed by Adjorlolo and Rao. Mims and co-workers were the first to suggest that mechanisms that involved the formation of C-O-K complexes on carbonaceous surface were an essential step of catalysis as this could determine the activity of the catalyst. Other authors proposed the formation of a nonstoichiometirc oxide with excess alkali metals that attracted oxygen from gaseous reactants and electrons from the carbon matrix to enhance the reaction of the adsorbed atoms. Further studies affirmed the presence of oxygen on the carbon surface to be essential for keeping the catalyst active, thus suggesting oxygen to be involved with the catalytic process. In their investigation, Zahedi and Miller also proposed surface oxygen groups to be of paramount importance to the catalytic process, thus suggesting the interactions between the catalyst and carbon to be the dominating steps during the carbon gasification process. According to Mims and co-workers who studied the potassium-catalysed gasification of graphite, potassium salts reacted readily with the carbon to form surface salt complexes. K2CO3 was found to spread across the surface of graphite in its active mode. The same authors also reported a strong interaction between the catalyst and the graphitic edges, this being comparable with cohesive bonding within the bulk of the material. Such strong interfacial forces were thought to be responsible for the observed dispersion of the catalyst along the graphitic active edges. The oxidation of graphite was reported to proceed by recession of the layer planes. Most of these studies show evidence of some sort of interaction or bonding between the carbon and the potassium and sodium carbonate catalysts. This is also evident in the case of the catalytic oxidation of C-C composites. Fig.2 is from the work of the present authors
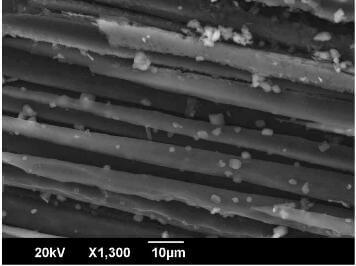
potassim carbonate particles attached to carbon fibers following oxidation of a C-C composite for 3h at 600C
during catalytic oxidation of C-C blocks at 600C and shows adhesion of K2CO3 particles on carbon fibers. In addition, fig.2 also reveals that the K2CO3 particles had melted or were generated from a molten phase even though the temperature of oxidation was well below the melting points of K2CO3 and K2O. With this in mind, it is important to consider the research of McKee and Chatterji who investigated the reactions between alkali metal carbonates and oxides with graphite in oxygen, carbon dioxide and helium atmospheres via the use of thermogravimetric and differential thermal analysis over a temperature range between 25C and 1000C. The alkali metals studied included rubidium, caesium, lithium, potassium and sodium all of which were found to catalyse the oxidation reaction of graphite. Catalytic activity commenced well below the melting point of the corresponding metal monoxide and interestingly around the melting point of their respective peroxides. Scanning electron microscopy indicated the presence of carbonate particles on the graphite. The carbonate particles appeared to have undergone melting even though again the oxidation temperature was below the melting temperature of the carbonate. By using thermogravimetry, McKee and Chatterji also showed that the dissociation of the metal carbonate to its oxide could not take place at temperatures below 500C. However, when the metal carbonate was mixed with graphite, the reaction
M2CO3 + C + O2 → M2O + 2CO2 (7)
became favourable. These authors also observed the conversion of the monoxides to peroxides by means of an exothermic reaction which was followed by peroxide melting below 500C. By using chemical thermodynamic data as compiled by Turkdogan, it can be shown that the dissociation of the carbonate at temperatures below 600C is only possible in the presence of carbon. The conversion of the monoxide to the peroxide can also be shown to be possible at similar temperatures. The observed catalytic effect of the alkali metals could not be attributed to the oxide-metal-oxide type of cycle that was proposed by Fox and White and by Adjorlolo and Rao due to the higher chemical thermodynamic stability of the peroxides at temperatures between 400C and 500C in comparison to the monoxides. By combining these observations, McKee and Chatterji proposed the following three-step redox cycle to account for the catalysed oxidation of graphite:
- Carbon-induced decomposition of the metal carbonate:
M2CO3 + C + O2 → M2O + 2CO2 (8)
- Oxidation of the metal oxide to a peroxide:
M2O + nO2 → M2O1+2n (9)
- Reduction of the metal peroxide to the oxide:
M2O1+2n + 2nC → M2O + 2nCO (10)
In the case of the alkali metals the value of n is 0.5. The catalytic effect was observed to increase going down the group in the Periodic Table due to the respective increased tendency to form the peroxide.