Carbon-carbon (CC) materials are a generic class of composites similar to the graphite/epoxy family of polymer matrix composites. These materials can be made in a wide variety of forms, from one-dimensional to n-dimensional, using unidirectional tows, tapes, or woven cloth. Because of their multiformity, their mechanical properties can be readily tailored. Carbon materials has high strength and stiffness potential as well as high thermal and chemical stability in inert environments. These materials must, however, be protected with coatings and/or surface sealants when used in an oxidizing environment.
The development of CC material began in 1958 and was nurtured under the U.S Air Force space plane program. It was not until the Space Shuttle Program that CC material systems were intensively researched. The criteria that
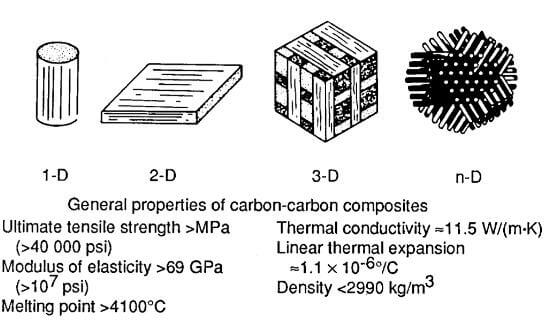
multiformit and general properties of carbon fiber and carbon matrix composite
led to the selection of CC composites are as a thermal protection system were based on the following requirements: (1) maintenance of reproducible strength levels at 1650C, (2) sufficient stiffness to resist flight loads and large thermal gradients, (3) low coefficient of thermal expansion to minimize induced thermal stresses, (4) oxidation resistance sufficient to limit strength reduction, (5) tolerance to impact damage, and (6) manufacturing processes within state of the art.
Carbon-carbon composites consist of a fibrous carbon substrate in a carbonaceous matrix. Although both constituents are the same element, this fact does not simplify composite behavior because the state of each constituent may range from carbon to graphite. Crystallographic carbon, namely graphite, consists of tightly bonded, hexagonally arranged carbon layers that are held together by weak van der Vaals forces. The single crystal graphite structure is illustrated in figure 2.
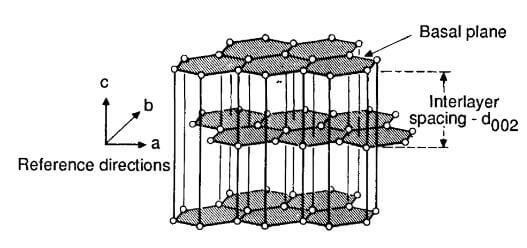
carbon layers held together by weak van der Waals forces.
The atoms within the layer plane or basal plane (a-b direction) have a covalent bond strength of about 524kj/mol, while the bonding energy between basal planes (c direction) is about 7 kj/mol. The result is a crystal that is remarkable in its anisotropy, being almost isostatic within the basal plane but with c direction properties that differ by orders of magnitude. On a larger scale, carbon, in addition to its two well-defined allotropic forms (diamond and graphite), can take any number of quasicrystalline forms ranging continuously from turbostratic to a highly crystalline graphite.
The anisotropy of the graphite single crystal encompasses many structural forms of carbon. It ranges in the degree of preferred orientation of the crystallites and influences the porosity, among other variables. A broad range of properties is the result of this anisotropy, which is available in carbon material. In CC composites, the range of properties can extend to both constituents. Coupled with a variety of processing techniques that can be used in the fabrication of CC composites, great flexibility exists in the design of and that resultant properties to be obtained from CC composites.
The wide range of properties of carbon materials can be shown when comparing the tensile moluli of commercially manufactured carbon fibers that range from 27.6Gpa to 690Gpa. In fabrication, the fibers can be used in either continuous or discontinuous form. The directionality of the filaments can be varied ranging from unidirectional lay-ups to multidirectional weaves. The fiber volume used constitutes another variable. The higher the volume fraction of a specific high-strength fiber in a matrix, the greater the strength of the composite. The matrix can be formed via two basic approaches: (1) through the carbonization of an organic solid or liquid, such as resin or pitch, and (2) through the chemical vapor deposition (CVD) of carbon from a hydrocarbon. A range of carbon structures can be obtained by either approach. Finally, heat treatment of the composite material at graphitization temperature offers additional variability to the properties that can be obtained. Typically, there is an optimum graphitization temperature at which the highest strength can be obtained fro a given composite composition of fiber and matrix.